We are excited to announce significant improvements to our Supermarket App Pro ingredient scanner! At Supermarket App Pro, we’re dedicated to continually enhancing our features based on your valuable feedback. One common concern we’ve heard is that our previous scanner’s strict rules occasionally led to missed ingredients. We’ve taken your feedback to heart and have implemented changes to make our scanner more comprehensive and accurate than ever before.
What’s Changed?
Previously, our scanner operated with strict rules, scanning for exact matches to ingredients in our database. While this ensured precision, it sometimes resulted in overlooked ingredients, leaving our users feeling less confident in their purchases. In response, we’ve revamped our approach.
How Does It Work Now?
Our updated scanner now employs a more flexible methodology. Instead of exclusively seeking exact matches, it comprehensively scans all the ingredients listed on a product label and compares them to our extensive database. This means that every ingredient present will be considered, potentially resulting in multiple matches if we have several entries for the same ingredient.
Why the Change?
We made this adjustment to address the concerns raised by our users. By adopting a more inclusive approach, we aim to provide you with a more thorough and accurate analysis of the ingredients in the products you’re considering. Your health and safety are our top priorities, and we’re committed to delivering the best possible experience with Supermarket App Pro.
What Does This Mean for You?
With our enhanced scanner, you can shop with greater confidence, knowing that our app is diligently scanning all listed ingredients to identify any potential concerns. Whether you’re managing allergies, dietary restrictions, or simply striving for healthier choices, our scanner is here to support you every step of the way.
Get Started Today!
Ready to experience the upgraded Supermarket App Pro ingredient scanner? Simply update your app to access the latest features and start enjoying a more comprehensive and reliable ingredient analysis.
We Value Your Feedback
Your feedback is what drives us to improve, so please continue to share your thoughts and suggestions with us. Together, we’ll continue to refine our features and make Supermarket App Pro the ultimate tool for informed shopping decisions.
Thank you for choosing Supermarket App Pro. Happy scanning!

Fevi became an advocate for food is medicine when her dog, Junior, was diagnosed with cancer. In that journey, she discovered how food played such an important role, first in animal health and then eventually, her own health. When she became a Mom, it reinforced the importance of healthy, natural and fresh food. She is a researcher, by profession, and her skills have helped in intimately understanding how the food industry has prioritized profits over health. As the Founder of Source Partners, she hopes to support the next evolution (perhaps, revolution) of the food industry.

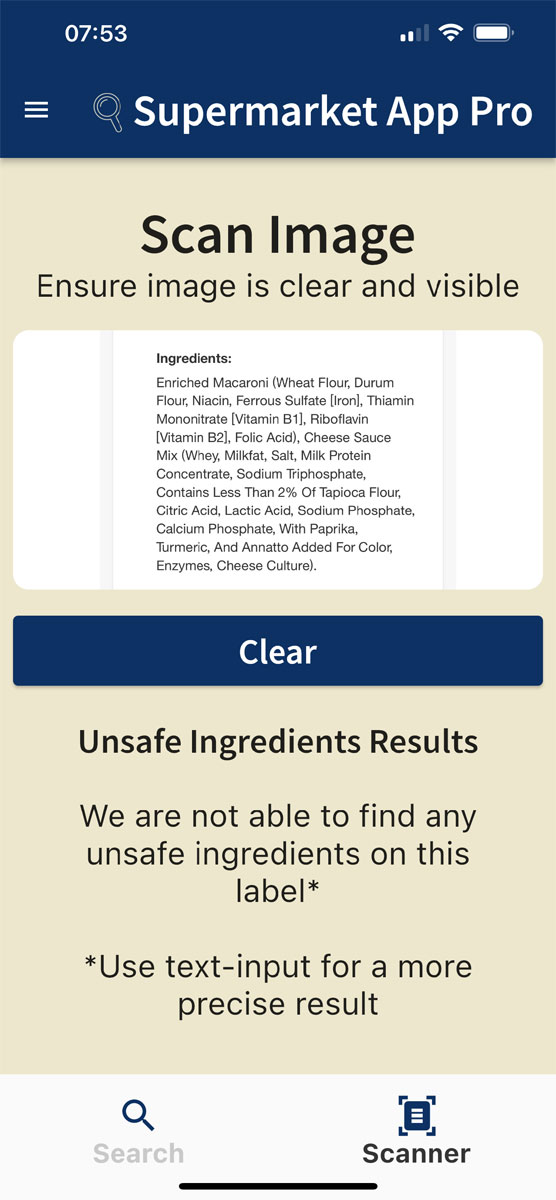 Here is the ingredient label of Kraft’s Mac and Cheese:
Here is the ingredient label of Kraft’s Mac and Cheese: